
Shanghai Henlius Biotech, Inc. (Henlius) has recently received an investigational new drug (IND) application acceptance notification from the China Drug Administration (CDA) for HLX12, a recombinant fully human monoclonal antibody against the vascular endothelial growth factor receptor-2 (VEGFR2), for the treatment of advanced gastric cancer and gastroesophageal junction (GEJ) adenocarcinoma non-small cell lung cancer (NSCLC) and metastatic colorectal cancer (mCRC).
HLX12 is a biosimilar of ramucirumab developed by Henlius. By binding to the VEGFR2, HLX12 works as a receptor antagonist blocking the binding of vascular endothelial growth factor (VEGF) to VEGFR2. VEGFR2 is known to mediate a complex cascade of the downstream VEGF signaling pathways that result in angiogenesis. VEGF blockade inhibits these signaling pathways and thereby effects the tumor survival, migration and invasion.

As of today, there is no approved anti-VEGFR2 monoclonal antibody in mainland China. Since the cost for a single treatment of ramucirumab is expensive, Henlius expects to develop HLX12 to potentially increase market competition and treatment accessibility of anti-VEGFR2 antibody.
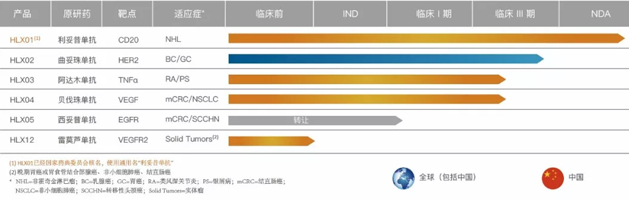
Apart from strengthening the bio-novel pipeline and combination immuno-oncology therapy, Henlius also has developed a robust biosimilar portfolio. Henlius first four biosimliars are currently in Phase 3 clinical stage, with additional products currently investigating at the pre-clinical phase.
Biosimilar Pipeline of Henlius
About Henlius
Founded in 2009, Shanghai Henlius Biotech, Inc. (Henlius) is a global clinical-stage company focusing on the discovery, development, manufacturing and commercialization of high-quality and innovative biologics to treat a range of chronic and life-threatening diseases. With a team of over 600 employees in Shanghai, Beijing, Taipei and Bay Area (CA), Henlius has established an integrated global product development platform.

Henlius has a robust pipeline of biosimilar and bio-novel products, including 11 clinical-stage candidates to treat cancer and autoimmune diseases. Henlius has completed 24 successful IND/CTA filings (14 approvals from China; 3 from the United States; 3 from Taiwan; 1 from the European Union; 1 from Ukraine; 1 from Philippines and 1 from Australia).