Shanghai, China, September 18, 2024 – Shanghai Henlius Biotech, Inc. (2696. HK) announced that the results on its first innovative product HANSIZHUANG (serplulimab) as well as its trastuzumab biosimilar approved in China, Europe and U.S., HANQUYOU were released as poster presentations at the 2024 European Society of Medical Oncology (ESMO) Congress.
HANSIZHUANG (serplulimab) is a recombinant humanised anti-PD-1 monoclonal antibody(mAb)injection independently developed by Henlius, and the world's first anti-PD-1 mAb approved for the first-line treatment of SCLC. The product has been approved in China, Indonesia, Cambodia and Thailand. Underpinned by the patient-centric strategy, Henlius has carried out a differentiated and multi-dimensional layout in the field of gastrointestinal cancer and lung cancer, covering a wide variety of indications. Up to date, HANSIZHUANG has been approved by the National Medical Products Administration (NMPA) for the treatment of MSI-H solid tumours, sqNSCLC, ES-SCLC, and esophageal squamous cell carcinoma (ESCC), benefiting about 80,000 patients. Meanwhile, HANSIZHUANG’s Marketing Authorisation Application (MAA) for ES-SCLC has been validated by the European Medicines Agency (EMA), which is expected to be approved in 2024. Moreover, a wide variety of clinical trials on immuno-oncology combination therapies in differentiated indications has been initiated by the company to further explore the efficacy of the product, such as HANSIZHUANG plus bevacizumab and chemotherapy as first-line treatment for patients with metastatic colorectal cancer (mCRC) , HANSIZHUANG plus chemotherapy as neoadjuvant/adjuvant therapy for gastric cancer (GC), and HANSIZHUANG plus chemotherapy and concurrent radiotherapy in patients with limited-stage small cell lung cancer (LS-SCLC), etc.
HANQUYOU is the trastuzumab biosimilar independently developed by Henlius in accordance with the National Medical Products Administration (NMPA), the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA) and other international biosimilar guidelines. It is Henlius’ first FDA-approved product. HANQUYOU is indicated for the treatment of HER2-positive early breast cancer, metastatic breast cancer and metastatic gastric cancer, which corresponds to all the approved indications of the trastuzumab originator. Up to date, HANQUYOU has been approved in 48 countries and regions, benefiting 210,000+ patients, bringing affordable and high-quality treatment options to breast cancer and gastric cancer patients worldwide.
ASTRUM-005

Title
Smoking-related genomic mutation patterns in patients with small cell lung cancer treated in ASTRUM-005 study
Methods
ASTRUM-005 was a randomized, double-blind, placebo-controlled, global, phase 3 trial in patients with extensive-stage SCLC.
Smoking signature analysis
Genomic mutations in 302 patients with available baseline tumor samples were assessed by the Med1CDx panel, which included exon regions of 601 genes. Two bioinformatic methods, the transversion/transition ratio (TTR) method and the Catalogue of Somatic Mutations in Cancer (COSMIC) Signature 4 method, were applied to analyze tobacco-smoking–related signature.
For the TTR method: An R Bioconductor package, Maftools, was used to calculate the fraction of transversion and transition in each sample. TTR value was then defined as the transversion fraction divided by the transition fraction in each sample[2-3]. Specifically, Maftools classified single nucleotide variants (SNVs) into 6 different transition and transversion events (C>A:G>T, C>G:G>C, C>T:G>A, T>A:A>T, T>C:A>G, T>G:A>C). Synonymous SNVs were included in these analyses.
For the Signature 4 method: Mutational signatures were extracted and the contributions of tobacco-smoking–related signatures (Signature 4), annotated by the COSMIC, from the genomic mutation panels of each patient were estimated[4].6 R package “deconstructSigs” was used to calculate the contribution of the mutation[5]. Both methods were validated on targeted panel sequencing from a published NSCLC data set. Both methods were able to distinguish smokers from non-smokers in NSCLC[6].
Statistical analysis
For progression-free survival (PFS) and overall survival (OS), the median was calculated from product-limit (Kaplan–Meier) estimates, while n was the number of patients in each subgroup category. The hazard ratio (HR) and its 95% confidence interval (CI) were estimated using an unstratified Cox proportional hazards model; Efron’s method was used to handle ties. For the analyses of genomic-based smoking signatures, the Wilcoxon test was used to calculate the difference among patients with different smoking histories. The clinical data cutoff date was June 13, 2022.
Results
Baseline characteristics in smoking groups
Patients in the SCLC cohort (N = 302) were grouped based on their smoking history; 23% were current smokers, 55% former smokers, and 22% never smokers. Baseline patient characteristics are summarized in Table 1.
Mutation analysis demonstrated that the most frequently mutated genes were TP53 (90% and 91%), RB1 (68% and 69%), and LRP1B (31% and 28%) in current and former smokers versus never smokers, respectively. Differential analysis on the mutated genes demonstrated that smokers and non-smokers have similar top mutated genes, with never smokers having more mutation in EGFR, DMD, MED12, MTOR, NOTCH1, REL, PGR, DNMT1, GRM3, KMT2A, and CD22.
Smoking signatures in patients with SCLC
No significant differences in smoking signatures were found between groups with different smoking histories using both methods (TTR: P = 0.54; Signature 4: P = 0.38) .
Findings were validated by applying the same analyses on 2 cohorts’ data sets profiled with whole exome sequencing from published studies[1,6]. In 120 samples from 40 patients with SCLC, no correlation between mutation pattern and smoking history in either method was observed (TTR: P = 0.91; Signature 4: P = 0.70)[7].
We observed similar results in another independent data set with whole exome sequencing performed on 110 samples from patients with SCLC (data not shown).10 Patients were further grouped into high/low TTR groups using the median TTR of 1.12 as the cutoff value. The mutation analysis showed that REL was more frequently mutated in non-smokers (P < 0.001) .
Higher TTR resulted in shorter median OS (HR [95% CI], 1.65 [1.05-2.62]; P = 0.03) for patients who only received chemotherapy, while both the high and low TTR groups gained similar benefits for patients who received serplulimab plus chemotherapy (HR [95% CI], 0.97 [0.67-1.4]; P = 0.87) .
Patients in both treatment groups (chemotherapy and serplulimab plus chemotherapy) showed similar benefits in PFS, regardless of the TTR (HR [95% CI], 1.25 [0.83-1.88]; P = 0.29 and 0.95 [0.67-1.34]; P = 0.77, respectively) .
Conclusion
In our ASTRUM-005 study, 67 (22%) patients with SCLC had never smoked. Unlike what was observed with NSCLC, patients with SCLC from our study showed similar tobacco-smoking–related genomic mutation patterns, regardless of their smoking status. Patients with SCLC who have never smoked may develop transversion mutations from other sources unrelated to direct tobacco exposure. Patients with high TTR might gain less benefit from chemotherapy, suggesting mutations in SCLC might be predictive biomarkers for certain therapies.
ASTRUM-004

Title
Exploratory biomarker analysis of phase 3 ASTRUM-004 study: serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer
Methods
Eligible patients were planned to be randomized (2:1) to receive serplulimab or placebo, both in combination with chemotherapy.
Genetic mutations were assessed by the Med1CDx panel in the biomarker evaluable population (BEP). The objective response rate (ORR) was compared between treatment arms and the odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using a stratified Cochran–Mantel–Haenszel method. Median PFS and OS were estimated using the Kaplan–Meier method. Comparisons between treatment arms were performed, and hazard ratios (HR) and their 95% CIs were estimated by a stratified Cox proportional hazards model.
Results
The clinical data cutoff date was January 31, 2023. A total of 537 eligible patients were enrolled (358 patients in the serplulimab group and 179 patients in the placebo group); 309 patients with detectable samples were included in the BEP.
Baseline characteristics and gene mutation status
Baseline characteristics and efficacy in the BEP were comparable with those in the ITT.
Mutation analysis demonstrated that the most frequently mutated genes were TP53 (85%), LRP1B (34%), and KMT2D (28%), consistent with published data[8-10].
Clinical outcomes by mutation status
In order to identify predictive biomarkers for serplulimab treatment, we analyzed genes and signaling pathways with different mutation frequencies in responders (patients showing complete response or partial response) and non-responders (patients with stable disease or progressive disease) to serplulimab plus chemotherapy treatment. Mutations in the Notch signaling pathway and several other genes were identified. The ORR was consistently higher in patients treated with serplulimab versus placebo, regardless of gene mutation status. A trend of greater ORR was observed in patients treated with serplulimab who had these mutations.
Mutations in the Notch signaling pathway, especially for NOTCH3, were associated with longer median PFS in patients receiving serplulimab versus placebo, possibly due to their roles in the tumor microenvironment in sqNSCLC, which was consistent with previous findings.[11] Mutations in KMT2D, which is involved in the modulation of chromatin structure, and PIK3C2G and EPHA3, which regulate the tumor microenvironment, were associated with better clinical outcomes in the serplulimab arm compared with the placebo arm.
Conclusion
The exploratory biomarker analysis suggests improved clinical benefit with the addition of serplulimab to chemo regardless of genetic mutation status. Furthermore, comparing to those without mutations, patients with mutations in Notch signalling pathway, KMT2D, PIK3C2G, or EPHA3 may derive more clinical benefit when serplulimab was added.
ASTRUM-LC01

Title
Consolidation Serplulimab Following Concurrent Hypofractionated Chemoradiotherapy for Limited-stage SCLC: Preliminary Analysis of Phase II ASTRUM-LC01 Study
Methods
LS-SCLC patients aged between 18-75 years with ECOG performance score of 0-1 were enrolled.
Treatment regimens:
-Chemotherapy: etoposide, 100 mg/m2 d1-3+, cisplatin, 75 mg/m2 d1, or carboplatin, AUC = 5 d1; Q3W, up to 4 cycles
-Hypofractionated radiotherapy: 45Gy/3Gy/15F
-Prophylactic cranial irradiation: 25Gy/2.5Gy/10F
-Consolidation therapy: serplulimab, 300 mg Q3W, until progression or unacceptable toxicity or up to 1 year
The preliminary analysis aimed to evaluate the efficacy of serplulimab consolidation therapy on the objective response rate (ORR), depth of response (DpR), disease control rate (DCR), survival outcomes, and safety profile in LS-SCLC patients.
Results
Patients
Between May 2022 and August 2023, 55 patients were enrolled. The date of data cut-off was April 7, 2024, and the median follow-up duration since initiating serplulimab was 9.8 months. The median cycles of consolidation therapy were 8, and 9 (16.4%) patients had completed the prescribed treatment.
Efficacy
The ORR and DCR following serplulimab treatment were both 96.4% (95% CI 87.5-99.6). The tumor responses were assessed following serplulimab treatment compared with pre-chemoradiotherapy.
Overall, 90.9% of patients achieved a DpR exceeding 50%, and patients achieved sustained tumor remission following serplulimab consolidation therapy.
The median progress-free survival (PFS) was not reached, and 1-year PFS rate was 71.8% (95% CI 60.1-85.8).
Safety
Four patients discontinued the study due to TRAEs. Pneumonitis (5.45%) was the most common grade 3-4 TRAEs. No treatment-related death event was reported.
Conclusion
These results highlight the potential survival benefit and manageable safety of consolidation serplulimab therapy in LS-SCLC.
A Real-world Retrospective Analysis

Title
Efficacy and Safety of Integrating Consolidative Thoracic Radiotherapy with Immunochemotherapy in ES-SCLC: A Real-world Retrospective Analysis
Study Design
This single-center retrospective study analyzed the medical records of patients diagnosed with ES-SCLC at Shandong Cancer Hospital between January 1, 2022 and December 31, 2023. Patients were stratified into three cohorts based on the first-line treatment received: chemoradiotherapy (cohort A), immunochemotherapy (cohort B), and immunochemotherapy followed by cTRT (cohort C). Propensity score matching (PSM) was utilized to adjust for baseline differences. Primary outcomes include real-world PFS (rwPFS) and OS, and secondary outcomes include real-world safety profile.
Results
Of the 374 patients analyzed, cohort C showed significant improvements in rwPFS and OS compared to cohort A. The median rwPFS in cohort C (10.9 months) was longer than cohort A (7.6 months) and B (8.0 months). The 12-month rwPFS rate was highest in cohort C (41%), compared to cohort A (19%) and cohort B (34%). Median OS (mOS) was 14.0 months in cohort A, 20.8 months in cohort B, and not reached in cohort C, with the lower limit of the 95% CI for cohort C being 17.80 months. The 18-month OS rate in cohort C (63%) was numerically higher than both cohort A (28%) and B (62%). After propensity score matching, cohort C still showed improvements in rwPFS and OS. The incidence of grade 3 or higher adverse events was comparable across cohorts, with myelosuppression being the most common. However, the incidence of grade 3 or higher pneumonitis was notably higher in cohorts B and C, aligning with previous reports.
Conclusion
Over half of the patients in the immunotherapy cohorts (B and C) received serplulimab (an anti-PD-1 monoclonal antibody). The combination of cTRT with immunochemotherapy for ES-SCLC showed improved rwPFS and OS, and the overall safety profile remained manageable. These findings highlight the need for further prospective studies to confirm the optimal integration of cTRT in ES-SCLC treatment strategies.
An Open-label, Single-arm, Phase 2 Trial
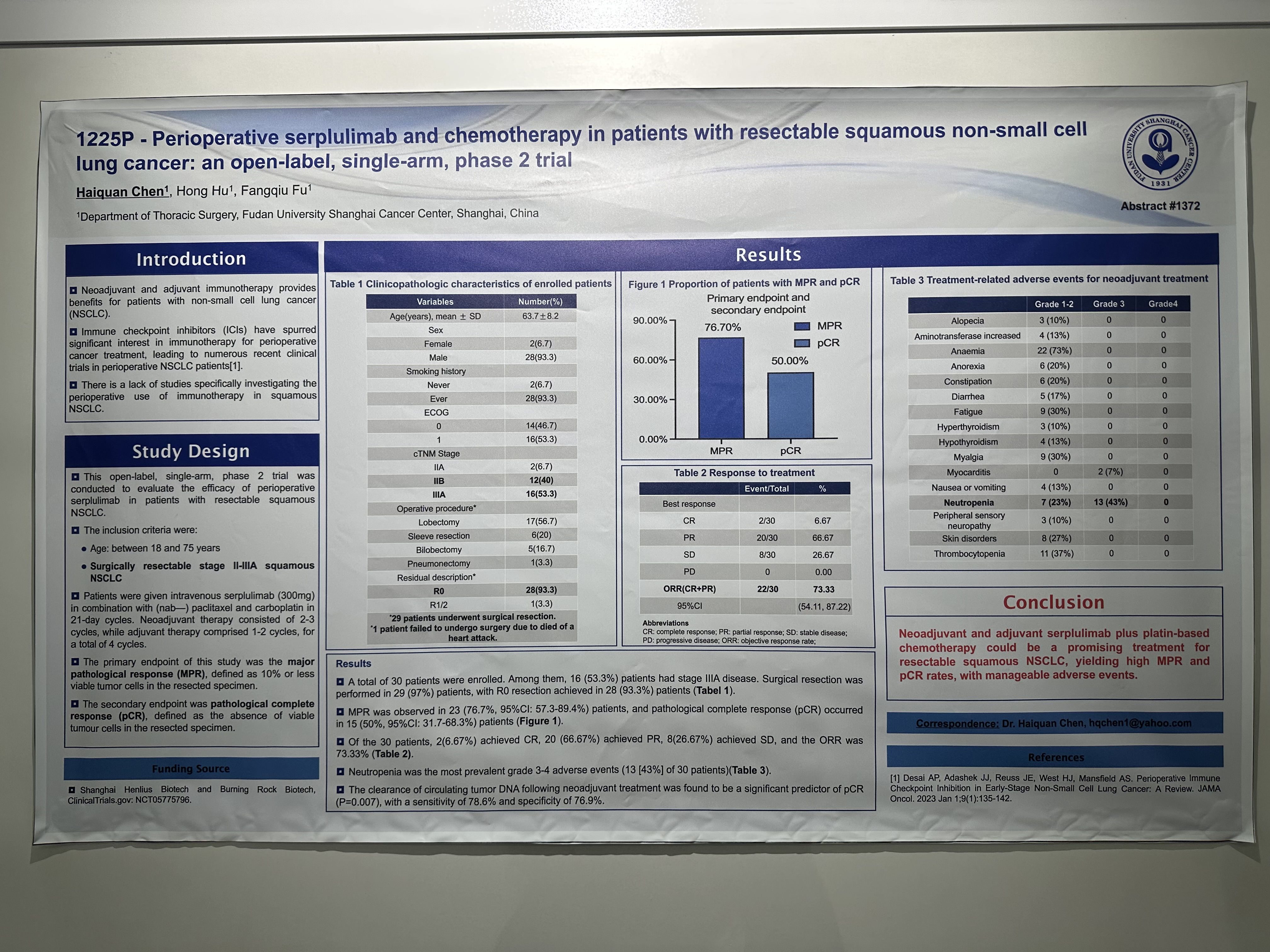
Title
Perioperative serplulimab and chemotherapy in patients with resectable squamous non-small cell lung cancer: an open-label, single-arm, phase 2 trial
Study Design
This open-label, single-arm, phase 2 trial was conducted to evaluate the efficacy of perioperative serplulimab in patients with resectable squamous NSCLC.
The inclusion criteria were:
-Age: between 18 and 75 years
-Surgically resectable stage II-IIIA squamous NSCLC.
Patients were given intravenous serplulimab (300mg) in combination with (nab—) paclitaxel and carboplatin in 21-day cycles. Neoadjuvant therapy consisted of 2-3 cycles, while adjuvant therapy comprised 1-2 cycles, for a total of 4 cycles.
The primary endpoint of this study was the major pathological response (MPR), defined as 10% or less viable tumor cells in the resected specimen.
The secondary endpoint was pathological complete response (pCR), defined as the absence of viable tumour cells in the resected specimen.
Results
A total of 30 patients were enrolled. Among them, 16 (53.3%) patients had stage IIIA disease. Surgical resection was performed in 29 (97%) patients, with R0 resection achieved in 28 (93.3%) patients.
MPR was observed in 23 (76.7%, 95%CI: 57.3-89.4%) patients, and pathological complete response (pCR) occurred in 15 (50%, 95%CI: 31.7-68.3%) patients.
Of the 30 patients, 2(6.67%) achieved CR, 20 (66.67%) achieved PR, 8(26.67%) achieved SD, and the ORR was 73.33%.
Neutropenia was the most prevalent grade 3-4 adverse events (13 [43%] of 30 patients).
The clearance of circulating tumor DNA following neoadjuvant treatment was found to be a significant predictor of pCR (P=0.007), with a sensitivity of 78.6% and specificity of 76.9%.
Conclusion
The exploratory biomarker analysis suggests improved clinical benefit with the addition of serplulimab to chemo regardless of genetic mutation status. Furthermore, comparing to those without mutations, patients with mutations in Notch signalling pathway, KMT2D, PIK3C2G, or EPHA3 may derive more clinical benefit when serplulimab was added.
Neoadjuvant Treatment in HER2-positive, Stage II-III Breast Cancer
Title
Phase II study of pyrotinib plus albumin-bound paclitaxel and Trastuzumab (HLX02) as neoadjuvant treatment in HER2-positive, stage II-III breast cancer
Methods
Patients with previously untreated non-metastatic HER2-positive BC were assigned to receive six neoadjuvant cycles of oral pyrotinib (400 mg) once daily, plus HLX02 (8 mg/kg loading dose, followed by 6 mg/kg) and Nab-P (260 mg/m2) q3w. The primary endpoint was pCR (ypT0/isN0). CTCs was captured and counted at baseline (P0), after neoadjuvant therapy (P1), and after surgery (P2) using Metafer-SE-iFish, and analyzed using Imaris 10.1 following image acquisition with the BZ-X800 microscope.
Results
From December 2020 to August 2023, a total of 214 patients were enrolled and completed neoadjuvant therapy and surgery. The pCR rate was 62.96% (95% CI, 55.65% -69.86%, p=. 001). The most common grade 3-4 adverse events were diarrhea (34.7%) and neutropenia (6.5%). Of 95 patients who underwent CTCs analysis, 63 (66.3%) achieved pCR. CTCs count was significantly reduced at P1 compared to that of at P0 (3.06±3.38 vs.0.84±1.53 FU/5ml, p<.001). Patients with pCR have larger nuclei size at P0 (95% CI, 1.02-1.38; P =. 04), less count (95% CI, 1.49-8.28; P =. 03) and lower membrane intensity (95% CI,0.93-0.98; P =. 03) at P1 to those with non-pCR (Table).
Conclusion
In women with HER2-positive, stage II-III BC, the neoadjuvant regimen pyrotinib plus Nab-P and HLX02 effectively promoted pCR of tumor and presented an acceptable tolerability. Personalized monitoring of CTCs during neoadjuvant therapy of early BC may aid in real-time assessment of treatment response and help predict pCR.
Reference
1. Rizvi H, et al. J Clin Oncol. 2018;36:1645.
2. Song K, et al. Transl Lung Cancer Res. 2018;7:439-449.
3. Mayakonda A, et al. Genome Res. 2018;28:1747-1756.
4. Alexandrov LB, et al. Nature. 2013;500:415-421.
5. Rosenthal R, et al. Genome Biol. 2016;17:31.
6.Zhou H, et al. Nat Commun. 2021;12:5431.
7.George J, et al. Nature. 2015;524:47-53.
8. Xu F, et al. Onomatology. 2020;9:1731943.
9. Yu J, et al. Cancers. 2022;14:3382.
10. Pan Y, et al. Cancer Cell. 2023;41:88-105.
11. Zhou C, et al. J Thorac Oncol. 2023;18:93-105.