Shanghai, China, March 14, 2024 – Shanghai Henlius Biotech, Inc. (2696. HK) announced that the first subject was dosed for a phase 1 clinical trial of HLX42 (NCT06210815), an investigational EGFR-Targeting antibody-drug conjugate (ADC), for the treatment of advanced/metastatic solid tumours. HLX42 was developed by the company based on the collaboration with MediLink Therapeutics and was approved for conducting clinical trial by the National Medical Products Administration (NMPA) and U.S. Food and Drug Administration (FDA). In December 2023, FDA granted Fast Track Designation to HLX42 for the treatment of advanced/metastatic non-small cell lung cancer (NSCLC) patients with disease progression on EGFR targeted therapies.
Epidermal growth factor receptor (EGFR) belongs to the receptor tyrosine kinases family and plays an important role in maintaining normal cell functions such as cell proliferation, differentiation and migration. Mutation or overexpression of EGFR is considered to be closely associated with the occurrence of various solid tumours including non-small cell lung cancer (NSCLC), colorectal cancer (CRC), etc. Studies have shown that targeting EGFR is a valid strategy for anticancer therapy [1]. Despite the great success of monoclonal antibodies targeting EGFR and 3rd generation EGFR Tyrosine kinase inhibitors (TKIs), there are still great unmet medical needs for effective therapies for patients who are refractory to current therapies or relapse after standard of care [2]. ADC technology has vastly advanced in the last several years and provide a new treatment option for tumour patients [3]. The EGFR-targeting ADC has the potential to overcome the resistance to EGFR monoclonal antibodies and EGFR TKIs, which may bring clinical benefits to more patients with advanced NSCLC/CRC who are resistant or refractory to standard EGFR targeted therapy.
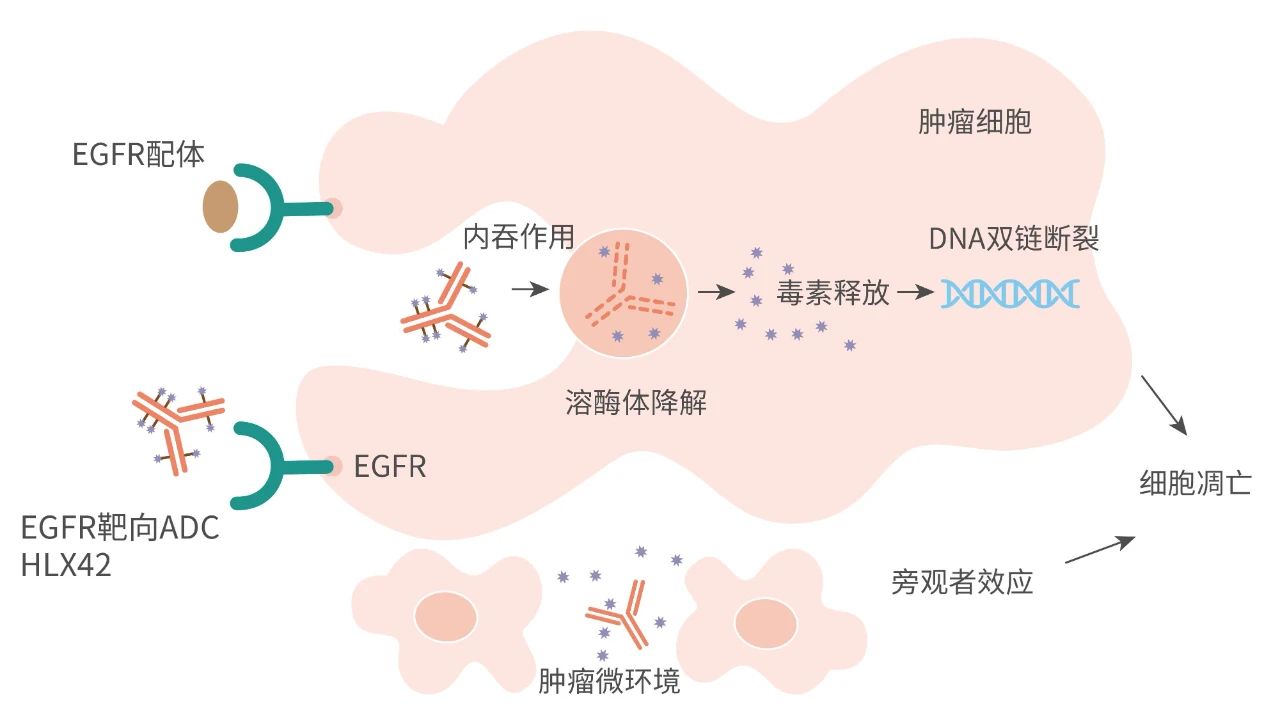
HLX42 is a novel EGFR-targeting ADC, comprised of a high-affinity humanised IgG1 mAb targeting EGFR conjugated with a novel cytotoxic payload through cleavable linkers, with the drug-to-antibody ratio is about 8. The cytotoxic payload is a novel DNA topoisomerase-I inhibitor which can cause double-strand breaks (DSBs) of DNA, block the replication machinery, thus trigger cancer cell apoptosis. When injected intravenously into the body, HLX42 linker-payload will be cleaved and released in tumour microenvironment (TME) with strong bystander killing effects. This unique mechanism of TME activation and payload release allows HLX42 to possess a higher therapeutic index and potency for treatment of solid tumours. HLX42 has exhibited good anti-tumour effects and a favorable safety profile in preclinical efficacy studies, pharmacokinetic studies and safety evaluation and the results were published as poster presentation at the 2023 European Society of Medical Oncology (ESMO) Congress. Meanwhile, HLX42 has shown potent tumour suppression in several CDX and PDX models that were EGFR TKIs or cetuximab resistant, which is expected to overcome the resistance of existing EGFR targeted therapies and fill the unmet clinical needs of more patients with advanced/metastatic solid tumours.
Underpinned by the patient-centric strategy, Henlius has pro-actively built a diversified and high-quality product pipeline, including about 60 molecules across monoclonal antibody (mAb), polyclonal antibody (pAb), ADC, fusion protein, and small molecule drug, of which more than 80% are self-developed. Regarding antibody technology as a core, Henlius will continue exploring novel targets and molecular mechanisms in more disease areas and conducting clinical studies for more innovative products to provide patients with quality and affordable biologics.
【参考文献】
[1] Ni cholson, Robert Ian, Julia Margaret Wendy Gee, and Maureen Elaine Harper. EGFR and cancer prognosis. European journal of cancer 37 (2001): 9-15.
[2] Tan, Chee Seng, D. Gilligan, and S. Pacey. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. The Lancet Oncology 16.9(2015): e447-59.
[3] Ponziani S, Di Vittorio G, Pitari G, et al. Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int J Mol Sci. 2020 Jul 31;21(15):5510.
About NCT06210815
This open-label, dose-escalation, first-in-human phase 1 clinical trial aims to evaluate the safety and tolerability of HLX42 in patients with advanced/metastatic solid tumours. Patients will receive HLX42 intravenously every three weeks at seven dose levels (0.1 mg/kg, 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, 2.0 mg/kg, 3.0 mg/kg, and 4.0 mg/kg) following a “3+3” design. The dose-limiting toxicity (“DLT”) observation period is three weeks after the first dose of HLX42. The primary endpoints of this study were the proportion of patients with DLT events in each dose cohort during the DLT observation period, and the maximum tolerated dose (MTD) of HLX42. Secondary endpoints include safety, pharmacokinetic parameters, immunogenicity, preliminary efficacy, pharmacodynamic measures, and potential predictive biomarkers and drug-resistance biomarkers.