Shanghai, China, November 24, 2023 – Shanghai Henlius Biotech, Inc. (2696.HK) announced that the first subject was dosed for a phase 1 clinical trial of HLX43, a novel PD-L1-targeting antibody-drug conjugate (ADC), for the treatment of advanced/metastatic solid tumours. HLX43 was developed by the company based on the collaboration with MediLink Therapeutics and is the first PD-L1-targeting ADC in China to enter a clinical trial.
Yongqiang Shan, general manager of Henlius’ Global Innovation Center, said: “I am delighted to be part of the HLX43 team as we achieve the dosing of the first subject in the phase 1 clinical trial. This milestone signifies Henlius’ transition from early development to clinical stage in ADC research. At Henlius’ Global Innovation Center, our unwavering commitment revolves around a patient-centric approach, addressing unmet clinical needs and focusing on providing patients with safer and more effective treatment. Looking ahead, we will continue to delve into novel modalities and diverse disease areas, leveraging the team's scientific expertise and collaborative spirit to illuminate the path forward for patients.”
PD-L1 is a trans-membrane protein considered to be a co-inhibitory factor of the immune response, it can combine with PD-1 to reduce the proliferation of PD-L1 positive tumour cells, inhibit their cytokine secretion and induce apoptosis [1]. Immune checkpoint inhibitors represented by PD-1/PD-L1 monoclonal antibodies have emerged in recent years and revolutionised all lines of treatment for tumour patients. However, there are still many patients with positive PD-L1 expression who do not respond to or develop resistance to PD-1/PD-L1-targeted therapy [2]. PD-L1 is expressed in patients across a broad spectrum of tumour types including non-small cell lung cancer (NSCLC), colorectal cancer(CRC), triple negative breast cancer(TNBC) and displays limited expression on normal tissues, highlighting the potential of PD-L1 as a target for ADCs in addition to its role as an immune checkpoint, which may bring promising effective treatment option for patients failed to benefit from PD-1/L1 immunotherapy [3].
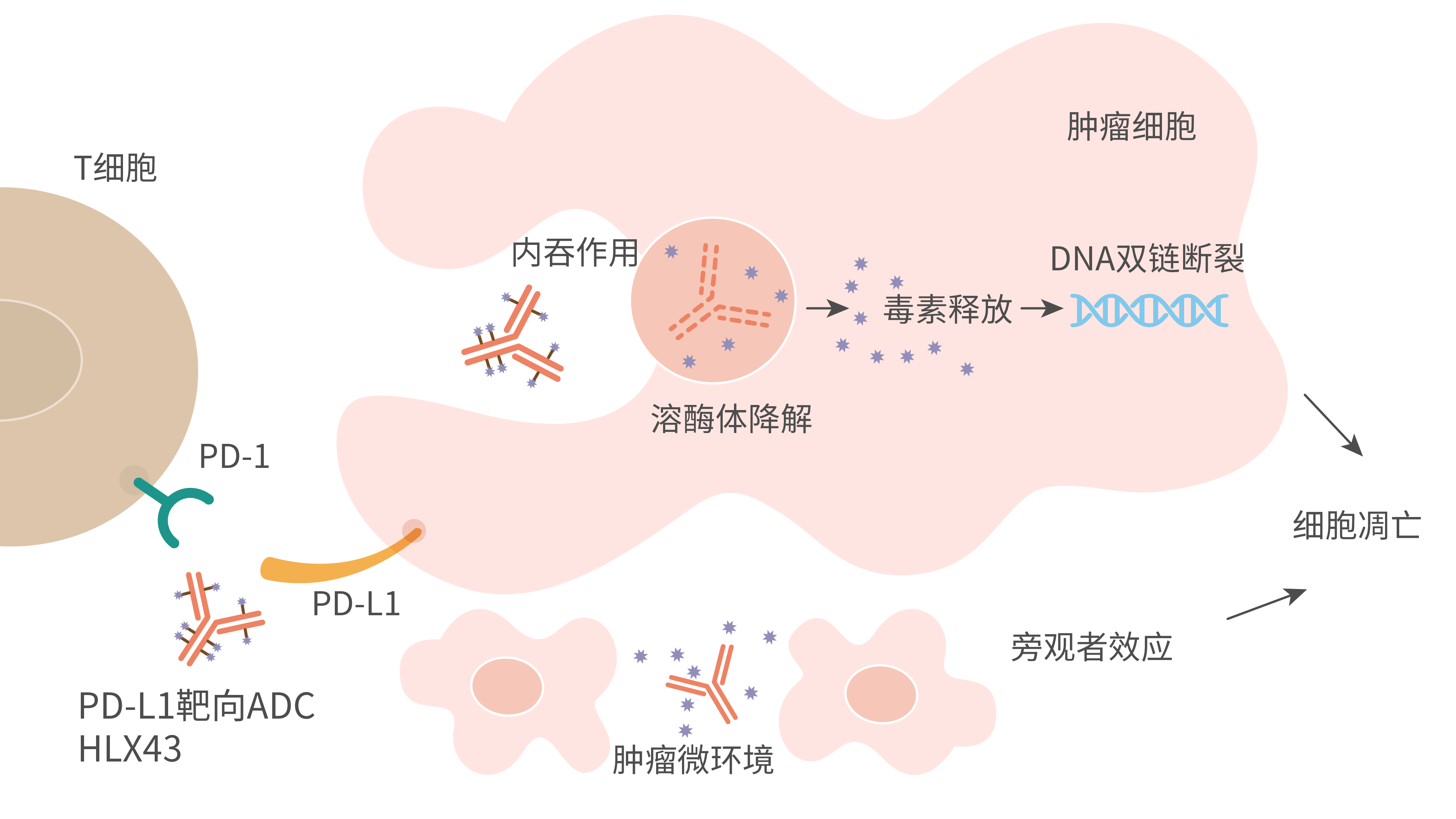
HLX43 is a novel PD-L1-targeting ADC, comprised of a high-affinity humanised IgG1 mAb targeting PD-L1 conjugated with a novel cytotoxic payload through cleavable linkers, with the drug-to-antibody ratio is about 8. The cytotoxic payload is a novel DNA topoisomerase-I inhibitor which can cause double-strand breaks (DSBs) of DNA, block the replication machinery, thus trigger cancer cell apoptosis. When injected intravenously into the body, HLX43 linker-payload will be cleaved and released in tumour microenvironment (TME) with strong bystander killing effects. This unique mechanism of TME activation and payload release allows HLX43 to possess a higher therapeutic index and potency for treatment of solid tumours. Several non-clinical studies have shown that HLX43 has good anti-tumour effects and favourable safety profiles and potent tumour suppression in several CDX and PDX models that were PD-1/L1 mAb resistant. Plus, the results were published as poster presentation at the 2023 European Society of Medical Oncology (ESMO) Congress.
Underpinned by the patient-centric strategy, Henlius has pro-actively built a diversified and high-quality product pipeline, including over 60 molecules across monoclonal antibody (mAb), bispecific antibody (BsAb), ADC, fusion protein, and small molecule drug conjugate, with emerging targets, including PD-1/L1, LAG-3, GARP, TIGIT, BRAF, etc. Regarding antibody technology as a core, Henlius will continue conducting clinical studies for more innovative products to provide patients with quality and affordable biologics.
About NCT06115642
This open-label, dose-escalation, first-in-human phase 1 clinical trial aims to evaluate the safety and tolerability of HLX43, in patients with advanced/metastatic solid tumours. The study will adopt a "3+3" design with six dose levels planned (0.5 mg/kg, 1 mg/kg, 2 mg/kg, 4 mg/kg, 6 mg/kg, and 8 mg/kg), and patients will receive different doses of HLX43 via intravenous infusion every three weeks. The dose-limiting toxicity (DLT) observation period is three weeks after the first dose of HLX43. The primary endpoints of this study were the proportion of patients with DLT events in each dose group during the DLT observation period, and the maximum tolerated dose (MTD) of HLX43. Secondary endpoints include safety, pharmacokinetic parameters, immunogenicity, preliminary efficacy, pharmacodynamic measures, and potential predictive biomarkers and drug-resistance biomarkers.
【参考文献】
[1] Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer[J]. American Journal of Cancer Research, 2020, 10(3):727-742.
[2] Attili I, Tarantino P, Passaro A, et al. Strategies to overcome resistance to immune checkpoint blockade in lung cancer[J]. Lung cancer: Journal of the International Association for the Study of Lung Cancer, 2021(154-):154.
[3] Kwan B, Ramirez M, Jin S, et al. 783 SGN-PDL1V, a novel, investigational PD-L1-directed antibody-drug conjugate for the treatment of solid tumors[J]. 2021.