Recently, Henlius has made a breakthrough in asymmetric bispecific antibody cell line development (CLD), and the study results have published in Biochemical Engineering Journal (link). Innovative CLD platform, established by CLD/PEX Director Yanling Wang, and the team from Henlus’ US Innovation Center, demonstrated a simple and effective way to obtain high-yield and high-quality cell lines for asymmetric antibody production. In this platform, over 90% of clones expressed the desired products, and stable monoclonal cell line increased the titer to 6g/L, such results have exceeded the industry's average level.
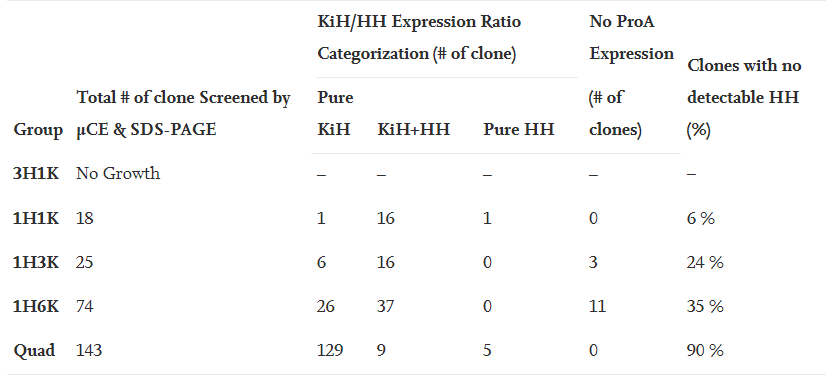
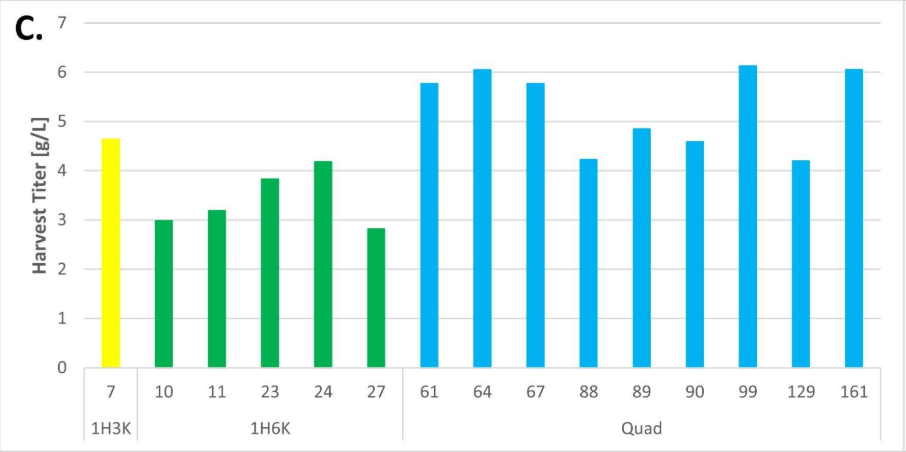
The stable cell line platform established by Henlius was significantly improved in purity, titer, and the percentage of single clones expressing desired products. The percentage of single clones expressing desired heterodimer was increased from 6% to 90%. The final titer of a single clone fed-batch production reached 6g/L with fewer product related impurities. Herein, an inspired CLD strategy was developed for asymmetric multichain bispecific antibodies, improving the overall screening efficiency and candidate clone quality and laying a firm foundation for innovative product development.
Henlius continues enhancing internal innovation capacities and external collaboration with more strategic partners. The two innovation centres of Henlius, located in China and the US work closely and support each other to enhance R&D efficiency. With antibody technology as the core, Henlius proactively explores immuno-oncology combination therapy, bispecific antibodies, and antibody-drug conjugates (ADC) to enrich diversified product pipeline, providing innovative and affordable medicines to meet global medical needs.