Shanghai, China, Nov, 24th, 2021 - Shanghai Henlius Biotech, Inc. (2696.HK) announced that the filing of a clinical trial for HLX301, a Recombinant Humanized Anti-PDL1 and Anti-TIGIT Bispecific Antibody, in Patients with locally advanced or metastatic solid tumours has been approved by the Bellberry Human Research Ethics Committee (“HREC”), and Clinical Trial Notification (“CTN”) has been acknowledged by the Therapeutic Goods Administration (“TGA”), Australia. The Phase 1 clinical study of the project in Australia is intended to be initiated in the near future. At present, no bispecific antibody targeting PD-1/PD-L1 and TIGIT has been approved for marketing globally.
Immunotherapies targeting immune checkpoint protein interactions between ligands and receptors have offered a novel way to attack tumour cells in recent years. Current research on immune checkpoint inhibitors is focused on CTLA-4 (cytotoxic T lymphocyte associated antigen-4) antibody, PD-1 (programmed cell death protein 1) antibody, and PD-L1 (programmed cell death-ligand 1) antibody. PD-1/PD-L1 plays a vital role in immune suppression; PD-1 and PD-L1 inhibitors show significant effectiveness in cancer treatment and have been approved for treating melanoma, non-small cell lung cancer, hepatocellular carcinoma, classical Hodgkin lymphoma, etc. TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domains) is an inhibitory receptor, mainly expressed on natural killer (NK) cells and activated CD8+ T cells, CD4+ T cells, and T regulatory cells. TIGIT binds to the major ligand CD155 (poliovirus receptor, PVR)1-2, mainly expressed on antigen-presenting cells (APC) or tumour cells, to down-regulate T cell and NK cell functions. As an inhibitory receptor, TIGIT can inhibit innate and adaptive responses in various mechanisms of action and act as a “brake” like PD-1/PD-L1 does to stop T cells from attacking tumours. Several pre-clinical studies have indicated that TIGIT blockade may be effective against multiple advanced cancers, including non–small-cell lung cancer, gastric cancer, melanoma, and multiple myeloma.
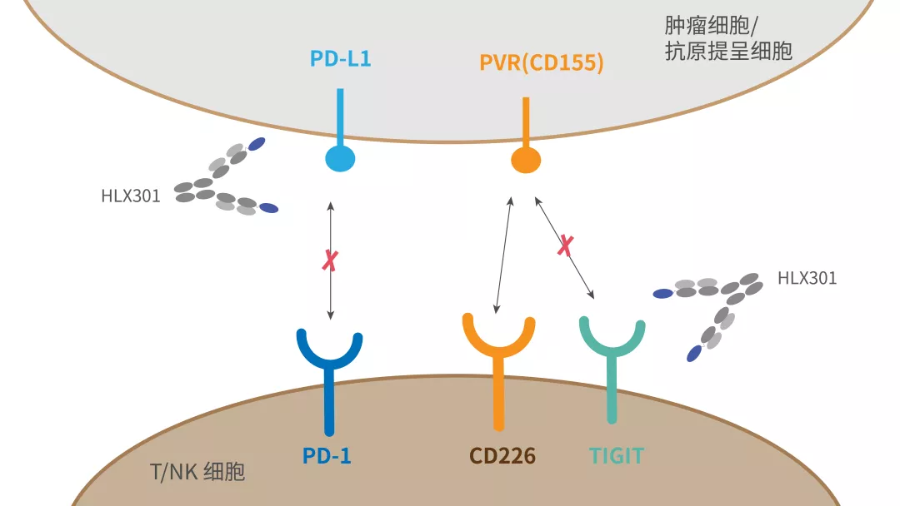
Independently developed by Henlius, HLX301 is an innovative anti-PD-L1 and anti-TIGIT bispecific antibody, composed of an anti-PD-L1 IgG1 subtype monoclonal antibody coupled with an anti-TIGIT variable domain of heavy-chain antibody. Its TIGIT binding domain is derived from VHH fragments with high affinity and high specificity to TIGIT, selected from the company’s synthetic humanized llama VHH library. Pre-clinical studies reported that HLX301 can simultaneously block both PD-1/PDL1 and TIGIT/PVR pathways, restore TCR signaling, inhibit tumour growth, and has good tolerance and safety. These results suggested that HLX301 is superior to blocking either pathway alone or anti-PD-L1 + anti-TIGIT combinational therapy, thus paving the way for further clinical development.
Underpinned by the patient-centric strategy, Henlius has achieved an overall layout of the immune checkpoint products of PD-1/L1, CTLA-4, LAG-3, etc., proactively exploring immuno-oncology combination therapy. The company has also built an innovative product pipeline with many emerging targets, including c-MET, TROP2, BRAF, etc. and has been developing a forward-looking presence in bispecific antibodies and the antibody-drug conjugates (ADC). Looking forward, Henlius will continue its momentum for innovation, further strengthening the in-licensing and collaboration on external innovative assets, and bringing more high-quality and affordable therapies to patients worldwide.
Reference
[1] Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2).
[2] Sanchez-Correa B, Valhondo I, Hassouneh F, et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers (Basel). 2019;11(6):877.